Background:
Outcomes of adults with relapsed/refractory acute myeloid leukemia (RR-AML) have remained poor. CD38 is a transmembrane glycoprotein that is highly expressed in AML and is potentially targetable with CD38 blocking agents [Naik J et al. Haematologica 2019, Tembhare et al. J Immunother Cancer 2020]. XmAb18968 is a novel CD38-CD3 bi-specific T-cell engager with Fc domain modified to minimize Fcγ receptor binding and non-selective T-cell activation resulting in reduced cytokine release without compromising target cell killing. We designed a phase I clinical trial to assess the safety and tolerability of XmAb18968 in patients with RR-AML.
Methods:
We conducted a multicenter, open label, phase I dose escalation study of XmAb18968 (NCT05038644). Patients aged ≥18 years with relapsed/refractory AML, ECOG PS 0-2 and adequate organ function were eligible. Major exclusion criteria are hematopoietic cell transplantation within 6 months of enrollment, active acute graft-versus-host disease, and acute promyelocytic leukemia. Dose escalation was conducted using 3+3 design and 4 separate dose levels were studied [0.8mg, 1mg, 1.3mg, 1.5mg]. XmAb18968 was administered intravenously on days 1, 8, 15, 22 on a 28-day cycle. During cycle 1 alone, day 1 dose was fractionated and administered over 2 days to minimize the risk of significant cytokine release syndrome (CRS). Treatment was continued until progression or unacceptable toxicity. The primary objective was to determine the safety and dose limiting toxicity (DLT) and secondary objective was to determine the preliminary efficacy. Measurable residual disease (MRD) status was assessed using multicolor flow cytometry with 0.01% sensitivity. Correlative studies included pharmacokinetics, characterizing changes in serum cytokines and phenotypic expression of activated T-cells and leukemic cells, correlation of the phenotypic expression with changes in transcriptome at the single-cell level, proteomics evaluation of cytokine secretion at the single-cell level and correlation of response with N-glycan profiling, quantitative site-occupancy, and direct glycopeptide analysis.
Results:
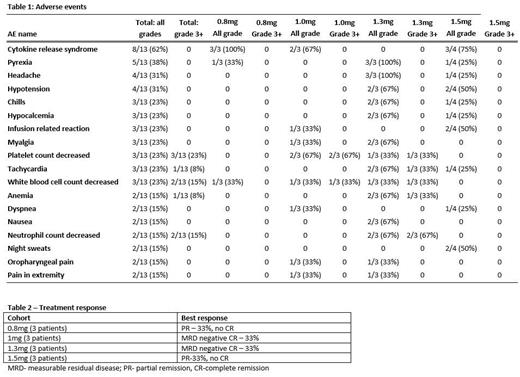
Thirteen patients with RR-AML were enrolled, 12 of whom were evaluable (1 patient came off study before DLT period). Median age was 63 years (45-77), 77% with adverse risk disease, 38% with secondary AML, and received median two (range 2-7) prior lines of therapy. Twelve of 13 patients (92%) were previously treated with venetoclax based regimen and 31% had prior allogeneic HCT. Adverse risk mutations included TP53 (31%), RUNX1 (23%), and ASXL1 (15%).
Grade 3 or higher adverse events included anemia (8%), neutropenia (15%), and thrombocytopenia (23%) (Table 1). CRS grades 1-2 were noted in 62% of patients, no grade ≥3 CRS or neurotoxicity (any grade) was seen. There was no dose limiting toxicity (DLT). Of the 12 evaluable total patients, clinically meaningful response was noted in two patients who were MRD positive by flow cytometry prior to study entry and achieved MRD negative CR (Table 2). These two patients who achieved MRD negative CR subsequently proceeded to allogeneic HCT. Median time to response was 1 cycle. Median OS of the cohort was 6.2 months (1.8-NR).
Conclusions:
XmAb18968 is safe and well tolerated in patients with RR-AML. Dose escalation yielded MRD negative CR in RR-AML patients who were MRD positive at study entry. Details on correlative studies examining mechanisms of therapeutic efficacy and resistance will be reported in the main meeting.
Disclosures
Guru Murthy:Amgen: Consultancy; Cardinal Health: Honoraria, Other: Advisory panel; BeiGene: Honoraria, Other: Advisory panel; Kite: Honoraria, Other: Advisory panel; Pfizer: Honoraria, Other: Advisory Panel; Rigel: Speakers Bureau. Leonard:Takeda: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite/Gilead: Consultancy; Pfizer: Consultancy. Shah:Moffitt Cancer Center: Current Employment; DSMC, Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences: Honoraria; Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite/Gilead: Other: Travel, Accommodations, Expenses; Incyte, Jazz Pharmaceuticals, Kite/Gilead, SERVIER: Research Funding; Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, Pepromene: Consultancy. Baim:Takeda: Research Funding; Novartis: Research Funding. Atallah:Abbvie: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal